FDA approves first self-administered flu vaccine spray
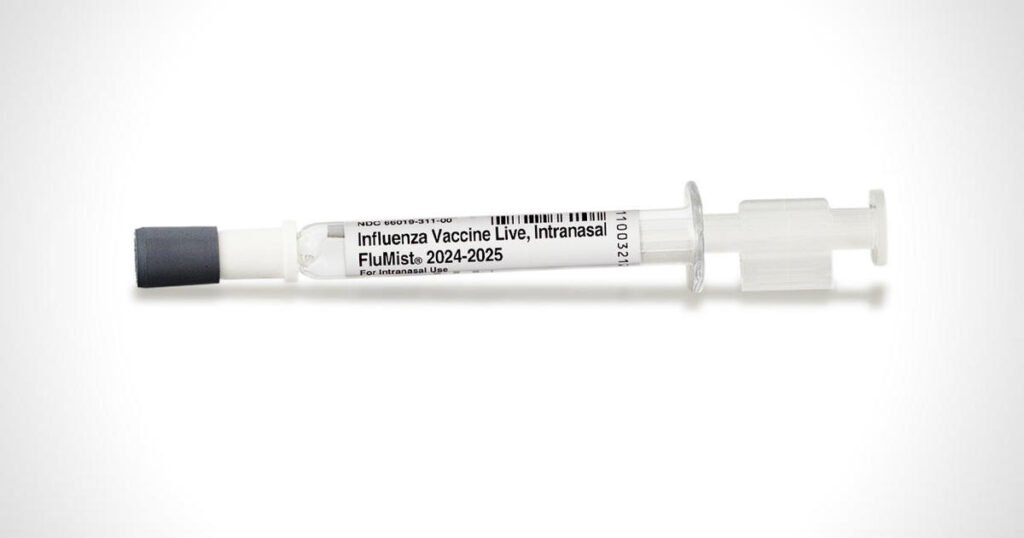
The Food and Drug Administration announced Friday it had broadened the approval of the FluMist nasal spray to become the first “self-administered” influenza vaccine — though a delay in the change means the vaccine will not be available to ship to homes until next year’s flu season at the earliest.
“Today’s approval of the first influenza vaccine for self- or caregiver-administration provides a new option for receiving a safe and effective seasonal influenza vaccine potentially with greater convenience, flexibility and accessibility,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
The FluMist vaccine, manufactured by AstraZeneca, had previously been approved back in 2003 to be given by health care providers similar to other flu shots. Now the vaccinemaker has approval to sell FluMist to adults for use at home on themselves or to administer to their children.
The FDA says patients will still need to get a prescription for the vaccine from a health care provider.
AstraZeneca says it plans to sell FluMist directly to patients through an online pharmacy. Adults will be able to complete a screening questionnaire to get the prescription, and then order shipments to their home.
There are also some limits to the kinds of people FluMist is recommended for. Since it uses a live but weakened version of the virus, some patients, like pregnant people or those who are severely immunocompromised, should not get this vaccine.
AstraZeneca
FluMist is less commonly used these days by pharmacies and doctors, in part due to fallout from a Centers for Disease Control and Prevention recommendation in 2016 against use of the spray over “poor or relatively lower effectiveness” compared to other vaccines.
AstraZeneca later redesigned the antigens in the vaccine, earning back the CDC’s recommendation starting in 2018.
Since then, the CDC says it has not had enough data for new official effectiveness estimates comparing FluMist to other flu vaccines, “because of limited use” in the U.S.
But AstraZeneca has cited data showing the shot has had “comparable” effectiveness in Europe versus more widely used shots.
AstraZeneca had initially told investors it hoped the FDA would broaden approval in time for this flu season, after the company submitted data last year showing that adults were able to correctly follow instructions to administer the vaccine spray on their own.
AstraZeneca did not comment when asked why the FDA’s approval decision came later than the company previously said it expected.
“We’re working diligently to bring this ‘first-of-its-kind’ innovative and convenient self-administrated flu vaccine to consumers and look forward to launching FluMist Home as soon as next flu season,” a spokesperson for the company said in a statement.
The spokesperson said AstraZeneca needed time to work with its partners to “ensure a seamless customer experience” for FluMist’s rollout for home use.
You may be interested

U.S.S. Eisenhower captain fights false Houthi claims with social media
new admin - Sep 21, 2024U.S.S. Eisenhower captain fights false Houthi claims with social media - CBS News Watch CBS News Houthi rebel groups claim…

Nick Saban blames Panthers for Bryce Young’s struggles: ‘Did not’ have talent around him
new admin - Sep 21, 2024[ad_1] Join Fox News for access to this content You have reached your maximum number of articles. Log in or…

Arizona Supreme Court rules that 98,000 people without confirmed citizenship docs can still vote in state races
new admin - Sep 21, 2024The Arizona Supreme Court ruled Friday that nearly 98,000 people whose citizenship documents hadn't been confirmed can vote in state…